Prevent Potential Life Sciences Corruption and Fraud
The state of the life sciences industry shows manufacturing hubs and the potential for corruption and fraud in those locations.
COVID-19 has sparked multiple discussions around moving device and pharma production, currently concentrated in Asia, back to the US, or diversifying it across several global locations to mitigate supply chain risks during global emergency situations such as the 2019 pandemic.
 The US government has asked pharmaceutical and medical device manufacturers to try and find a more sustainable domestic production model to prevent shortages of critical supplies such as masks, gloves, medical gowns, and ventilators. A search for new manufacturing partners comes with a need to vet new entities against multiple global watchlists to prevent fraud, money-laundering, or corruption risks.
The US government has asked pharmaceutical and medical device manufacturers to try and find a more sustainable domestic production model to prevent shortages of critical supplies such as masks, gloves, medical gowns, and ventilators. A search for new manufacturing partners comes with a need to vet new entities against multiple global watchlists to prevent fraud, money-laundering, or corruption risks. Alternatively, pharmaceuticals and medical device companies could diversify their production across other global production hubs to prevent over-reliance on any one particular foreign country for the life-saving drugs and devices. The need to vet these new entities protects businesses and ensures a strong corruption defense tool to also support regulatory responsibilities.
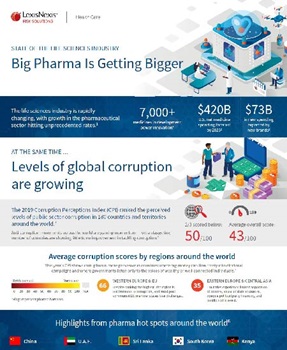
Download the 2020 State of Life Sciences infographic for the current industry snapshot. Find out where the main manufacturing hubs are and the level of corruption of the countries in which they are located. The information contained in this infographic can help protect your company’s brand and bottom line.
Access Full Infographic
